Immunochromatography Guide
Immunochromatography assay (ICA), namely lateral flow test, is a simple device intended to detect the presence or absence of the target analyte. The concept of immune-chromatography is a combination of chromatography (separation of components of a sample based on differences in their movement through a sorbent) and immunochemical reactions. The most widespread immunochromatographic system is the test strip (Figure 1).
Developing an ICA
Strips used for ICA contain four main components:
1. Sample Application Pad
It is made of cellulose and/or glass fiber and sample is applied on this pad to start the assay. Its function is to transport the sample to other components. Sample pad should be capable of transportation of the sample in a smooth, continuous and homogenous manner. This pretreatment may include separation of sample components, removal of interferences, adjustment of the pH, etc. analyte sample should be added to the sample application pad to start the test.
2. Conjugate Pad
It is the place where labeled biorecognition molecules (labeled antibodies, usually nano colloid gold particle) are dispensed. Material of conjugate pad should immediately release labeled conjugate upon contact with moving liquid sample. Labeled conjugate should stay stable over entire life span of the lateral flow strip. Any variations in dispensing, drying or release of conjugate can change the results of assay significantly. Poor preparation of labeled conjugate can adversely affect sensitivity of the assay. Glass fiber, cellulose, polyesters and some other materials are used to make conjugate pad.
3. Substrate(Nitrocellulose) Membrane
It is highly critical in determining sensitivity of ICA. Test and control lines are drawn over this piece of membrane. So an ideal membrane should provide support and good binding to capture probes (antibodies, etc.). Nonspecific adsorption over test and control lines may affect results of assay significantly, thus a good membrane will be characterized by lesser non-specific adsorption in the regions of test and control lines. Proper dispensing of bioreagents, drying and blocking play a role in improving sensitivity of the assay.
4. Adsorbent Pad
It works as sink at the end of the strip. It also helps in maintaining flow rate of the liquid over the membrane and stops back flow of the sample. Adsorbent capacity to hold liquid can play an important role in results of assay.
All these components are fixed or mounted over a backing card. Materials for backing card are highly flexible because they have nothing to do with ICA except providing a platform for proper assembling of all the components. Thus, backing card serves as a support and it makes easy to handle the strip.

Figure 1. Typical layout of a lateral flow test strip.
Major steps in ICA are:
- (i) Preparation of labeled antibody and capture antibody against target analyte;
- (ii) Immobilizing the labeled antibody onto conjugate pad, and the capture antibody onto the strip membrane to form the Test/Control line.
- (iii) Assembling of all components onto a backing card after dispensing of reagents at their proper pads.
- (iv) Add samples and buffer onto sample pad.
- (v) Wait the sample flow through the test and control line for 5-10min.
- (vi) Read the result when the color reveal.
ICA Format
Lateral flow assay basically combines a number of variants such as formats, biorecognition molecules, labels, detection systems and applications. There are three types of ICAs based on detection format, which are:
1. Sandwich Assay
In this assay format, label (Enzymes or nanoparticles or fluorescence dyes) coated antibody is immobilized at conjugate pad. This is a temporary adsorption which can be flushed away by flow of any buffer solution. A capture antibody against target analyte is immobilized over test line. A secondary antibody against labeled antibody is immobilized at control zone (Figure 2).
To start a test, sample containing the analyte is applied to the sample application pad and it subsequently migrates to the other parts of strip. At conjugate pad, target analyte is captured by the immobilized labeled antibody and results in the formation of analyte-labeled antibody complex. This complex now reaches to nitrocellulose membrane and moves under capillary action. At test line, analyte-labeled antibody complex is captured by another antibody which is primary to the analyte. Analyte becomes sandwiched between labeled and primary antibodies forming labeled antibody-analyte-primary antibody complex. Excess labeled antibody will be captured at the control zone by secondary antibody. Buffer or excess solution goes to absorption pad. Intensity of color at test line corresponds to the amount of target analyte and is measured with an optical strip reader or visually inspected. Appearance of color at control line ensures that a strip is functioning properly.
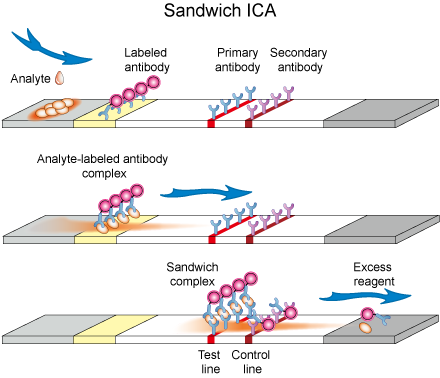
Figure 2. Schematic diagram of Sandwich ICA.
2. Competitive Assay
Competitive format has two layouts. In the first layout, solution containing target analyte is applied onto the sample application pad and prefixed labeled antibody gets hydrated and starts flowing with moving liquid. Test line contains pre-immobilized antigen (same analyte to be detected) which binds specifically to label conjugate. Control line contains pre-immobilized secondary antibody which has the ability to bind with labeled antibody. When liquid sample reaches at the test line, pre-immobilized antigen will bind to the labeled conjugate in case target analyte in sample solution is absent or present in such a low quantity that some sites of labeled antibody conjugate were vacant. Antigen in the sample solution and the one which is immobilized at test line of strip compete to bind with labeled conjugate (Figure 3.). In another layout, labeled analyte conjugate is dispensed at conjugate pad while a primary antibody to analyte is dispensed at test line. After application of analyte solution, a competition takes place between analyte and labeled analyte to bind with primary antibody at test line.
Such format suits best for low molecular weight compounds which cannot bind two antibodies simultaneously. Absence of color at test line is an indication for the presence of analyte while appearance of color both at test and control lines indicates a negative result.
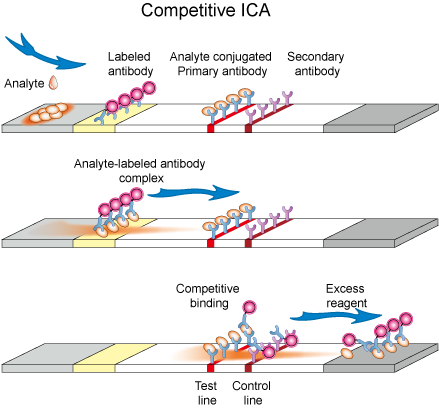
Figure 3. Schematic diagram of competitive ICA.
3. Multiplex Detection Assay
Multiplex detection format is used for detection of more than one target species and assay is performed over the strip containing test lines equal to number of target species to be analyzed. It is highly desirable to analyze multiple analytes simultaneously under the same set of conditions. Multiplex detection format is very useful in clinical diagnosis where multiple analytes which are inter-dependent in deciding about the stage of a disease are to be detected. Lateral flow strips for this purpose can be built in various ways, for example, by increasing length and test lines on conventional strip, making other structures like parallel threads, stars or T-shapes. Shape of strip for ICA will be dictated by number of target analytes.
Lateral flow immunoassays represent a well-established and very appropriate technology when applied to a wide variety of point-of-care (POC) or field use applications.
The advantages of the lateral flow immunoassay system (LFIA) are well known:
- Relative ease of manufacture – equipment and processes already developed and available. Easily scalable to high-volume production
- Stable – shelf-lives of 12-24 months often without refrigeration
- Ease of use: minimal operator-dependent steps and interpretation
- Can handle small volumes of multiple sample types
- Can be integrated with onboard electronics, reader systems, and information systems
- Relatively low cost and short timeline for development and approval
- Market presence and acceptance – minimal education required for users and regulators
Traditionally designed lateral flow immunoassays, however, have also suffered from performance limitations, most notably sensitivity and reproducibility. Some of these issues are listed below:
- Miniaturization of sample volume requirements below microliter level
- Multiplexing: simultaneous analysis of multiple markers difficult
- Integration with onboard electronics and built-in QC functions challenging
- Sensitivity issues in some systems
- Test-to-test reproducibility challenging – limits applications in quantitative systems
Benefit from its rapid test procedure and naked eyes visible characteristics, lateral flow immunoassays have achieved broad penetration in a variety of markets. Here we summarize some of the applications in figure 4.
Brows All Production Related to Rapid Test Kit
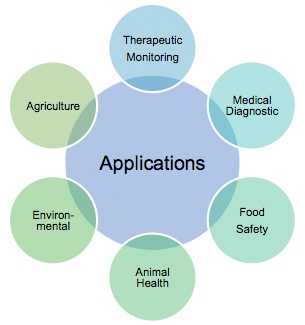
Figure 4. Applications of Immunochromatography assay.
Creative Diagnostics offers extensive experience in the development of rapid, point-of-care, lateral-flow-format diagnostic assays. We can fully develop an immunoassay test (or optimize an existing assay) according to your specifications. Once developed, we will ship the components to you, and all products associated with the project shall become the exclusive property of yours at the conclusion of the project. Full confidentiality is guaranteed.
Learn More about Colloidal Gold Lateral Flow Strips Development Service
References
- Dzantiev B B, Byzova N A, Urusov A E, et al. Immunochromatographic methods in food analysis[J]. TrAC Trends in Analytical Chemistry, 2014, 55: 81-93.
- Wong, R.C. and Tse, H.Y., Lateral Flow Immunoassay, New York: Humana Press, 2009.
- Zhou G, Mao X, Juncker D. Immunochromatographic assay on thread[J]. Analytical chemistry, 2012, 84(18): 7736-7743.
- Sajid M, Kawde A N, Daud M. Designs, formats and applications of lateral flow assay: A literature review[J]. Journal of Saudi Chemical Society, 2014.